Creating engaging, effective content can take a lot of effort, no matter what industry you’re in. In the Life Sciences industry, all of that work coordinating resources to produce on-brand, localized materials is just the first step of a long process of regulatory review and approval. Brand Managers in Life Sciences have a responsibility to adhere to that process in order to keep accurate records and to ensure their brands enjoy the complete confidence of patients and physicians.
The demands of regulatory agencies change from country to country, making the Medical Legal Regulatory (MLR) review process?a key step in content creation?a steep challenge. In the U.S., the FDA is the primary regulatory body that controls whether promotional materials for a new therapy can enter to the market. The FDA recently finalized its latest guidance, taking effect in June 2021, on how Life Sciences companies should submit their promotional materials for review. For that, the FDA requires companies to submit Form 2253 either electronically (eCTD) or in paper form.
Automatically prepare packages for submission to the FDA
What is FDA Form 2253? Form FDA 2253 is the standard form Life Sciences companies need to fill out to start the regulatory review process for their promotional content. Along with all the promotional content associated with a submission, the form gathers the information the FDA needs to review advertisements and promotions all in one place.
But getting that information together takes time and effort that many brand teams can’t afford to give. Most companies use third-party software or other services to help manage the actual submission process, but it’s the preparation of packages of promotional content for submission that are actually the most costly and time-consuming.
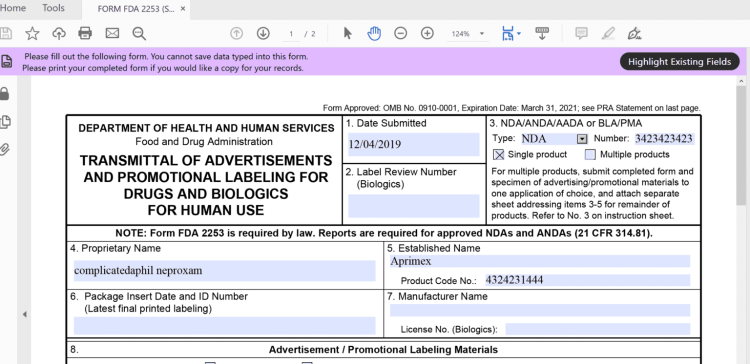
Aprimo helps enterprises with their FDA submission package. Aprimo fills out forms like Form FDA 2253 and packages the submission and will work with the tools you use to manage the final sending of materials to the FDA.
Aprimo helps users across the entire lifecycle of the content, from creation to completion to reuse. Aprimo deploys automatic preparation of review packages, collecting the necessary files, and automatically pre-filling forms like FDA 2253. This automation saves repetitive work and manual assembly of each package which can easily translate into hundreds of hours of work saved per month.
But Aprimo helps with everything leading up to the final package, too.
For example, the review and annotation process happens within Aprimo (you never have to leave the application) with a clear audit trail of who said what, approved when, and what they reviewed. Critical elements like the claims made in a piece of promotional material’such as ?new formula is 10% more effective?’legally must be backed up by scientific data that support the claim. During the annotation and markup phase of internal review, Aprimo enables users to include links to establish a connection between claims made in the promotional materials and the data that supports those claims. Once annotations are made, compliance needs continue to stack up and Aprimo streamlines all of it: e-signatures, two-factor authentication for voting on content, and personal PINs’saving firms a lot of time and money throughout the MLR preparation process.
Content is much more than MLR
Yes, MLR is a big concern. But there’s a twist to all of this:
Typically only about 20% of the content created by Life Sciences companies actually needs to go through the formal regulatory submission process.
That other 80% of content you’re creating? That’s where Aprimo has you covered.
Across the entire content lifecycle?from conception of an idea to planning, to distribution, localization, and reuse?Aprimo helps brands unleash the power of content to create meaningful, impactful, memorable experiences for patients and physicians. Whether or not that content requires regulatory submission, Aprimo gives you the power to realize the full value of your content.
With Aprimo, companies can centrally manage content to easily find it in their digital asset management system, adjust it for local markets, and share it across the globe, ensuring that every piece of content is on-brand and compliant.
The bottom line is that content is important. The content companies put into market?either via MLR or not?quite literally is the brand. It’s the voice of a brand and deserves to be taken as seriously as it deserves.
The way patients feel about therapies, whether physicians trust a brand at first sight?content can affect those things and make all the difference between someone choosing one therapy or another.